A different mechanism for causation of Alzheimer’s disease, with the protein aggregates now targeted therapeutically produced by downstream pathways
— Alan Herbert
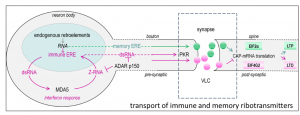
CHARLESTOWN, MA, UNITED STATES, September 20, 2024 /EINPresswire.com/ — One of the surprising recent findings was the connection between neurological plasticity and viral-like capsids encoded by endogenous retroelements (ERE). These capsids are capable of transmitting some RNAs across synapses, although the function of those ribotransmitters is not well understood.
Potentially the capsids can also transport immune active RNAs also derived from retroelements (sometimes called junk RNA), across the synapse to protect against the spread of viral pathogens. That mechanism, as supported by a number of previously published works, has the potential to interfere with memory formation.
The connection of these processes to Alzheimer’s disease is explained in this paper just out today. Many of the protein aggregation pathways that are the subject of much current scrutiny in Alzheimer’s disease are downstream of these effects.
In a paper published today in Frontiers in Neuroscience, Alan Herbert outlines the pathways involved. The endogenous retroelements (ERE) that are the focus of the manuscript derive from RNAs that just copy and paste themselves throughout the genome. These invasive elements comprise almost half of the human genome, whereas genes that make protein need for cellular function comprise 2,5%.
Over time, defenses have arisen to tame ERE to prevent their spread. Also, they have been modified to serve useful purposes. The best-known example is the use of an ERE protein to form placentas. Another example is the use of a different ERE protein to enhance neuroplasticity and promote memory formation. This memory EREs are capable of transporting certain RNAs across the synapse between neurons.
ERE RNAs are now used to defend the genome by marking RNAs as self. The recognition turns off the immune response against RNAs with such marks. The processes involve the formation of Z-RNAs that are encoded by ERE flipons. When expressed at high levels, the immune EREs instead turn on inflammatory pathways. They additionally turn off the translation of cellular RNAs into protein. Both processes limit the spread of viruses in the brain, they also can impair memory formation.
The paper describes how the interaction between memory and immune EREs are usually beneficial as they contribute to host survival. However, during chronic inflammation, the memory impairment becomes more pronounced, especially during neuroinflammatory diseases like Alzheimer’s. Other sequelae follow. For example, the failure to clear protein aggregates follows from those downstream effects that are aimed at preventing the cellular uptake of pathogens by neurons.
About InsideOutBio:
InsideOutBio is a start-up focused on developing a novel class of proprietary therapeutics to ‘light’ up tumors for the immune system to kill by reprogramming self/nonself pathways within cancer cells. Dr. Herbert leads discovery at InsideOutBio. His work on Z-DNA was foundational to the discovery of flipons.
These statements about InsideOutBio comply with Safe-Harbor laws. They are forward-looking and involve known and unknown risks and uncertainties. They are not guarantees of future performance and undue reliance should not be placed on them.
Alan Herbert
InsideOutBio, Inc
+1 617-584-0360
email us here
Visit us on social media:
X
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content “as is” without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()
Originally published at https://www.einpresswire.com/article/745173817/memory-loss-in-disease-from-neuroinflammation-due-to-junk-rna