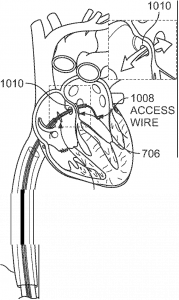
Distal disc retracts to cover the atrial septal defect (ASD), with the movable proximal disc positioned on the right atrial side.
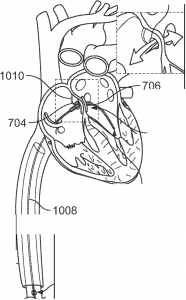
Proximal sphere covering the right atrial side of the atrial septal defect, with both spheres in place to close the ASD.
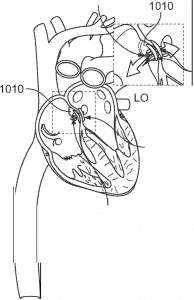
Deployed discs effectively closing the atrial septal defect.
Retriever Medical's innovative occlusion device for anatomical passageways has received Notice of Allowance, marking a major milestone in vascular technology. The issuance of the Notice of Allowance is a significant endorsement of our efforts to revolutionize the treatment of vascular diseases.”
— Ben Bobo LAS VEGAS, NV, UNITED STATES, September 10, 2024 /EINPresswire.com/ -- Retriever Medical, a leader in medical device innovation, is thrilled to announce that the United States Patent and Trademark Office (USPTO) has officially issued a Notice of Allowance for U.S. Application No. 17/809,528. This patent application covers a groundbreaking device designed for occluding anatomical passageways between structures, marking a significant advancement in medical device technology.
The patented technology promises to revolutionize the treatment of atrial septal defects and other anatomical passages. The device features a handle coupled to a tip portion via a first member, with a proximal element, a distal element, and a second member extending between them.
The key innovation allows the proximal element to move axially along the second member while the distal element remains fixed. Both elements can expand from a linear configuration to a three-dimensional geometric shape, precisely sealing anatomical passages.
Key Features and Advantages of the Patented Device:
• Expandable Elements: Proximal and distal elements can expand from a linear configuration to a significantly larger three-dimensional shape, enhancing occlusion effectiveness.
• Controlled Deployment: The device includes a handle with interfaces for precise control of the device's deployment and detachment, ensuring reliable placement and occlusion.
• Locking Mechanism: A unique locking device secures the device in place after deployment, with options for mechanical, electrolytic, or thermal release.
• Hydrogel Coating: Some elements are coated with hydrogel to assist in sealing and to adapt to anatomical passageways, improving effectiveness and patient outcomes.
• Versatile Use: The device can be employed for a range of anatomical passages and puncture sites, offering a significant improvement over conventional closure methods.
Additionally, the technology encompasses a thrombectomy system with a unique sensor for monitoring the pressure exerted on vessel walls by the expandable elements. This advancement ensures better control and minimizes complications related to conventional methods.
"The issuance of the Notice of Allowance is a significant endorsement of our efforts to revolutionize the treatment of vascular diseases. At Retriever Medical, we are driven by our mission to deliver innovative solutions that not only advance medical practice but also lead to better patient outcomes. We are excited to continue our journey with this innovative vascular approach," said Ben Bobo, CEO of Retriever Medical.
This Notice of Allowance highlights Retriever Medical's commitment to advancing medical technology and providing innovative solutions for challenging medical conditions. The approval reflects the device's potential to address critical needs in the medical field and improve patient care outcomes.
About Retriever Medical
Retriever Medical is a cutting-edge medical device company dedicated to developing innovative solutions for the treatment of vascular diseases. With a focus on mechanical thrombectomy, the company’s portfolio includes state-of-the-art devices designed to improve patient outcomes by providing physicians with advanced tools for the removal of blood clots. Retriever Medical is committed to advancing the field of interventional medicine through continued innovation and collaboration with leading healthcare professionals.
Ben Bobo
Retriever Medical, Inc.+1 7146542367email us hereVisit us on social media:XLinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
You just read:
News Provided By
September 10, 2024, 13:00 GMT
EIN Presswire's priority is author transparency. We do our best to weed out false and misleading content. The content above is the sole responsibility of the author who makes it available. If you have any complaints, kindly contact the author above. Originally published at https://www.einpresswire.com/article/741371313/retriever-medical-announces-notice-of-allowance-for-innovative-occlusion-device